If HCl is an acid, what would you expect the conjugate base to be? Why?
eleven.13: Cohabit Acid-Base Pairs
- Page ID
- 49522
Through examples found in the sections on acids and bases proton-transfer processes are broken into two hypothetical steps: (1) donation of a proton by an acid, and (two) acceptance of a proton past a base. (Water served equally the base in the acrid example and as the acid in the base example [amphiprotic]). The hypothetical steps are useful because they brand it easy to see what species is left after an acrid donated a proton and what species is formed when a base of operations accepted a proton. We shall utilise hypothetical steps or half-equations in this section, simply you lot should bear in listen that free protons never actually exist in aqueous solution.
Suppose we offset consider a weak acid, the ammonium ion. When information technology donates a proton to any other species, we can write the half-equation:
\[ \text{NH}_4^+ \rightarrow \text{H}^+ +\text{NH}_3\]
The submicroscopic representations below show the donation of the proton of ammonium. The removal of this proton results in NH3, which is easily seen at the submicroscopic level.
Just NH3 is ane of the compounds nosotros know as a weak base. In other words, when it donates a proton, the weak acid NHiv + is transformed into a weak base NH3. Another example, this time starting with a weak base of operations, is provided past fluoride ion:
\[\text{F}^{-} + \text{H}^{+} \rightarrow \text{HF}\]
The submicroscopic representation above shows how the addition of a proton to fluoride converts a weak base (F - in green) into a weak acid (HF).
The situation just described for NH4 + and NHthree or for F– and HF applies to all acids and bases. Whenever an acid donates a proton, the acrid changes into a base of operations, and whenever a base accepts a proton, an acid is formed. An acid and a base which differ only by the presence or absence of a proton are called a conjugate acrid-base pair. Thus NHthree is called the conjugate base of NH4 +, and NH4 + is the conjugate acid of NHiii. Similarly, HF is the conjugate acrid of F–, and F– the conjugate base of HF.
Instance \(\PageIndex{i}\) : Conjugate Pairs
What is the cohabit acrid or the cohabit base of (a) HCl; (b) CH3NH2; (c) OH–; (d) HCO3 –.
Solution:
- HCl is a strong acid. When it donates a proton, a Cl– ion is produced, and then Cl– is the conjugate base of operations.
- CH3NHii is an amine and therefore a weak base. Adding a proton gives CHthreeNH3 +, its conjugate acid.
- Adding a proton to the strong base OH– gives H2O its conjugate acid.
- Hydrogen carbonate ion, HCO3 –, is derived from a diprotic acrid and is amphiprotic. Its conjugate acid is HtwoCO3, and its conjugate base is COthree two–.
The use of conjugate acid-base pairs allows us to make a very simple statement about relative strengths of acids and bases. The stronger an acid, the weaker its conjugate base, and, conversely, the stronger a base, the weaker its conjugate acid.
Tabular array \(\PageIndex{1}\):Important Conjugate Acid-Base Pairs.
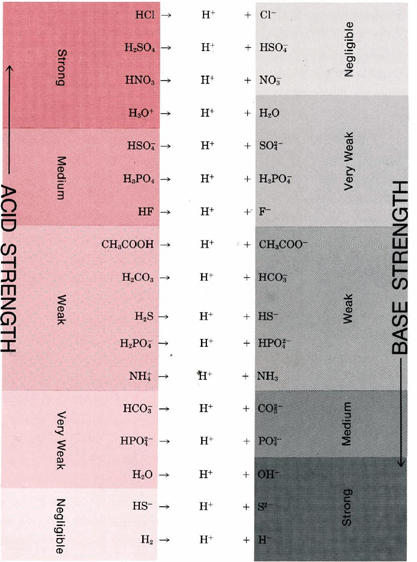
Table \(\PageIndex{1}\) gives a list of some of the more than of import conjugate acrid-base pairs in club of increasing forcefulness of the base. This tabular array enables u.s.a. to encounter how readily a given acid will react with a given base. The reactions with most trend to occur are between the strong acids in the tiptop left-hand comer of the table and the stiff bases in the lesser correct-hand comer.
If a line is fatigued from acid to base for such a reaction, it will have a downhill slope. Past contrast, reactions with little or no tendency to occur (between the weak acids at the lesser left and the weak bases at the pinnacle right) correspond to a line from acid to base of operations with an uphill slope. When the gradient of the line is not far from horizontal, the conjugate pairs are non very different in strength, and the reaction goes simply function way to completion. Thus, for instance, if the acrid HF is compared with the base CH3COO–, we expect the reaction to go part way to completion since the line is barely downhill.
A strong acid like HCl donates its proton then readily that in that location is essentially no trend for the conjugate base Cl– to reaccept a proton. Consequently, Cl– is a very weak base. A potent base like the H– ion accepts a proton and holds information technology so firmly that there is no tendency for the conjugate acid H2 to donate a proton. Hence, H2 is a very weak acrid.
Example \(\PageIndex{2}\) : Counterbalanced Equation
Write a balanced equation to draw the reaction which occurs when a solution of potassium hydrogen sulfate, KHSO4, is mixed with a solution of sodium bicarbonate, NaHCO3.
Solution
The Na+ ions and K+ ions accept no acid-base properties and function purely as spectator ions. Therefore any reaction which occurs must exist between the hydrogen sulfate ion, HSO4 – and the hydrogen carbonate ion, HCO3 –. Both HSOiv – and HCO3 – are amphiprotic, and either could human action as an acrid or as a base of operations. The reaction betwixt them is thus either
\(\text{HCO}_3^- + \text{HSO}_4^- \rightarrow \text{CO}_3^{2-} + \text{H}_2\text{SO}_4\)
or \(\text{HSO}_4^- + \text{HCO}_3^- \rightarrow \text{SO}_4^{2-} + \text{H}_2\text{CO}_3\)
Table \(\PageIndex{1}\) tells us immediately that the second reaction is the right 1. A line drawn from HSO4 – equally an acid to HCO3 – as a base is downhill. The first reaction cannot possibly occur to any extent since HCO3 – is a very weak acrid and HSO4 – is a base whose strength is negligible
Case \(\PageIndex{3}\) : Reaction Prediction
What reactions will occur when an excess of acetic acid is added to a solution of potassium phosphate, K3PO4?
Solution
The line joining CH3COOH to PO4 three– in Table \(\PageIndex{1}\) is downhill, and so the reaction
\(\text{CH}_3\text{COOH} + \text{PO}_4^{3-} \rightarrow \text{CH}_3\text{COO}^- \text{HPO}_4^{2-}\)
should occur. In that location is a further possibility because HPOiv 2– is itself a base of operations and might have a second proton. The line from CHiiiCOOH to HPOfour 2– is also downhill, but just barely, and so the reaction
\(\text{CH}_3\text{COOH} + \text{HPO}_4^{two-} \rightarrow \text{CH}_3\text{COO}^- + \text{H}_2\text{PO}_4^-\)
can occur, simply information technology does not get to completion. Hence double arrows are used. Although H2POfour – is a base and might be protonated to yield phosphoric acid, H3PO4, a line drawn from CHiiiCOOH to H2POfour – is uphill, and and then this does non happen.
Source: https://chem.libretexts.org/Bookshelves/General_Chemistry/Book:_ChemPRIME_%28Moore_et_al.%29/11:_Reactions_in_Aqueous_Solutions/11.13:_Conjugate_Acid-Base_Pairs

Post a Comment for "If HCl is an acid, what would you expect the conjugate base to be? Why?"